Medical device manufacturers are aiming to improve healthcare and automate surgical processes. The exponential leap in innovation made by medical device manufacturers and the quick adoption of their technology and products by the healthcare industry, has led to the need for ensuring that their products not only serve their purpose, but are also extremely safe and reliable.
Therapeutic temperature management and ventilation systems, defibrillation and circulation products are some of the avenues where medical device manufacturers are making inroads. Critical medical products must be tested to verify they meet transportation and operational vibration requirements. Sentek Dynamics collaborates with medical device manufacturers by conducting vibration testing on their products to help characterize their hardware. The Sentek Dynamics demo test lab located in Santa Clara, CA has a 50kN (11,000lbf) Model M5044A shaker used for testing a wide-variety of new medical devices.
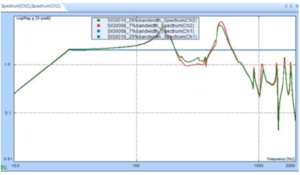
A Crystal Instruments’ Spider-81 Controller is used to run Sine sweeps to survey the products. Once the survey is completed, resonant modes are established to perform low and high frequency Sine dwells for fatigue and failure modes. The acceleration levels are increased in small increments to determine various modes of failure.